- Home
- / Pages
pH Adjustment
pH Adjustment
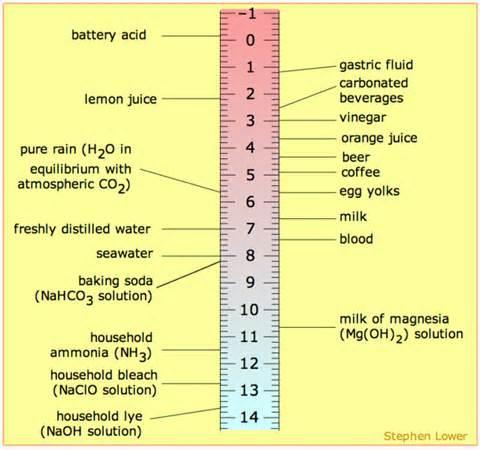
By Definition pH is the measure of free hydrogen activity in water. In more practical terms, although not technically correct in every case, pH is the measure of how acidic or basic a substance is. Measured on a scale of 0-14, solutions with a pH of less than 7 are considered acids while those with a pH of 7 or more are bases.
Bases are used to neutralize acids while acids are used to neutralize caustics; the term caustic and base, although not truly synonymous, are often exchangeable. The byproducts are normally salts and water.